Ideal Gas
- Concept: What is an ideal gas?
- Equation of state: The ideal gas law, PV = nRT,
or PV = NkT (you do not have to remember the
values of the constants R and k).
- Avogadro's number: The number of particles in a mole of ideal
gas, NA = 6.02
 1023. 1023.
Kinetic Theory
- Concept: What is kinetic theory?
- Main equation: Mean kinetic energy of a particle in an ideal
gas, mv2mean/2 = (3/2) kT.
First Law of Thermodynamics
- Concept: What is thermodynamics?
- Statement: Change in internal energy
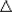 Esystem = Q + W,
where Q is the heat transferred to the system, and W the work done
on the system (by non-conservative forces). Esystem = Q + W,
where Q is the heat transferred to the system, and W the work done
on the system (by non-conservative forces).
- Work: Work done on a system with constant pressure W =
–P
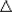 V. V.
Second Law of Thermodynamics
- Idea: In order to perform work, energy must flow between two
reservoirs set at different temperatures. By itself, heat always flows
from warmer to colder objects. The flow introduces disorder into the
system. Irreversible processes.
- Statements: In terms of direction of heat flow, and change
in entropy; Interpretation in terms of disorder and "organized
energy".
- Entropy: For an object at temperature T that absorbs
heat Q,
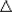 S = Q/T. S = Q/T.
|