Heat and Internal
Energy
- Heat: General concept, relationship with internal energy.
- Units: Definition of calorie (and kcal), and mechanical equivalent,
1 cal = 4.186 J.
Specific Heat and Heat of Transformation
(Latent Heat)
- Specific heat: Concept, Q = mc
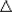 T, and application to calorimetry problems. T, and application to calorimetry problems.
- Heat of transformation: Concept, Q = mL,
and application to calorimetry problems.
Heat Transfer
- Mechanisms: Concepts of conduction, convection, and
radiation; What happens in each one; Be able to recognize situations
in which they apply.
|